EUCAST study highlights MASTDISCS® AST are of excellent and consistent quality
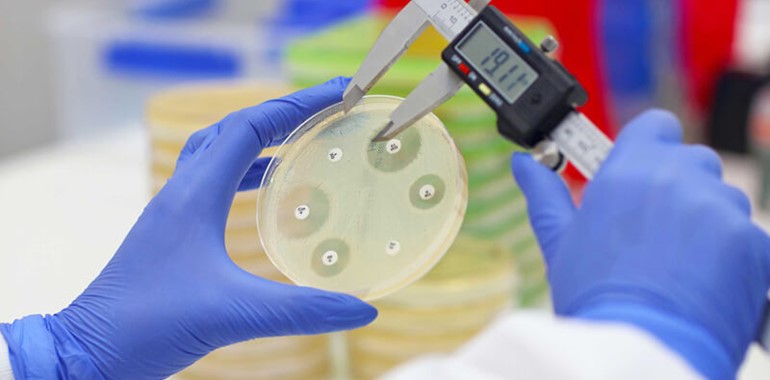
EUCAST evaluated the disc potency of 16 antimicrobial susceptibility test (AST) discs from nine manufacturers against EUCAST targets and ranges for relevant quality control strains.
The 16 AST discs were selected to represent different antibiotic classes and to include screening discs for important resistance mechanisms. The first study took place in 2014, on in-house prepared Mueller Hinton (MH) plates using MH agar from two manufacturers.
In 2017 EUCAST again invited manufacturers to participate in a repeat of these studies. The report highlighted that from 2014-2017 Mast Group Ltd. has maintained a high level of consistency of well calibrated discs maintaining both quality and performance throughout all studies.
The recently published paper by EUCAST continues this theme stating Mast Group Ltd.’s discs were found to ‘demonstrate excellent and consistent quality in both the 2014 and 2017 study’.
MASTDISCS® AST produced results 100% within the ranges set out by EUCAST and MASTDISCS® reproducibility continues to be of a high standard for all QC combinations (Ahman et al., 2018).
*Ahman J, Matuscheck E, Kahlmeter G. The quality of antimicrobial disks from nine manufacturers -EUCAST evaluations in 2014 and 2017. Clinical Microbiology and Infection (2018)
